Fusion in Stars (Proton-Proton cycle) and Mass-Energy
Nuclear fusion is the process which occurs in nature at the center of stars. The basics of this fusion are simple: two nuclei of hydrogen, or any
atom lighter than iron, collide at at high energies and fuse into heavier helium, or atoms no heavier than helium, atoms and release large amounts of energy.
In the case of stars, gravitational forces are responsible for consolidating the large clouds of hydrogen into bodies of extreme temperature and density
to allow fusion to occur. Because these perfect conditions exist in all stars, including our sun, they
essentially are nature's fusion reactors. Our best efforts to produce fusion on Earth will be somewhat similar, yet completely different, to the processes
that already occur in the center of the stars. It would be worthwhile, then, to examine in some detail the dominant fusion interaction within moderate
sized stars,the proton-proton fusion cycle (pictured to the right).
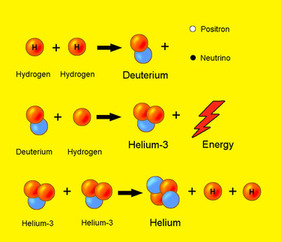
The diagram to the side represents the full process of proton fusion. Energy is a quantity that must be conserved, otherwise it would be possible to
construct a perpetual motion machine and nuclear fusion reactors would be altogether unnecessary. What exactly, then, is the origin of the energy yielded from
a fusion interaction? To answer this question in a very roundabout way, first draw your attention to the first stage in the proton-proton cycle pictured on the
diagram. After the two protons fuse, a complex process between subatomic particles called a weak interaction causes a proton to decay into a neutron, an electron neutrino,
and a positron for the nucleus to be as stable as possible.
Neutrinos are notoriously elusive subatomic particles of indeterminate mass and speed that are only known to interact with known forms
of matter via weak interactions. Fusion processes are driven by an entirely different fundamental force than the weak force, so this particle will continue on its
way to obscurity until the weak force calls upon it to either merge with a neutron to yield a proton and an electron or to be detected by researchers
and cause severe headaches. Positrons are particles similar to electrons, but with identical mass and opposite charge. While neutrinos tend to
react very little with any normal matter, positrons will do the exact opposite. Positrons have been observed to interact with electrons, which are
abundant in normal matter. When these particles meet, they annihilate each other such that they no longer exist. In the void left by the annihilation, there
are well documented observations that energy in the form of high-energy gamma ray photons travel in opposite directions. This phenomena, which can occur
due to proton-proton fusion in stars, is a direct demonstration of the mass-energy equivalence principle from Einstein's relativity.
Or...
E=mc^2
Binding Energy, Nuclear Forces, and Energy from Fusion
Whenever nuclei fuse and energy is released, an infinitesimal amount of mass disappears and is converted into energy. To restate this in a different
way, the total mass of each nucleon before fusion is greater than the mass of the resultant nucleus after fusion. This would be nonsensical if the
mass of an atomic nucleus was equal only to the total mass of its constituent protons and neutrons. To actually consider the mass of an atomic nucleus,
both the principle of mass-energy equivalence and the balance of an attractive force between nucleons that strongly against electromagnetic
repulsion between like-charged protons must also be considered.
When two light nuclei fuse together, such as Deuterium and Hydrogen binding to Helium-3 for example, the proton count and electromagnetic repulsion
has increased, but the nuclear radius is still small enough such that the increase in nucleon count increases the attractive force between nucleons
enough to totally overpower repulsion. For the fusing nuclei in this example the addition of a proton increases the attractive force between nucleons
yet the change in the repulsive force is negligible. The nucleus then becomes more tightly bound, or said differently, possesses a greater binding
energy than it did before the fusion. The binding energy of an atomic nucleus is a quantity representing the amount of energy required to separate
the nucleons against the attractive force holding them together, so the binding energy is an energy well represented a negative quantity of no
insignificant value.
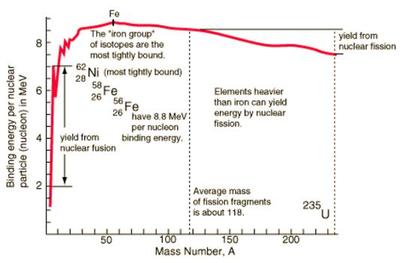
The energy invested by an atomic nucleus into its binding energy is taken from the mass-energy of its constituent protons and neutrons. The graph to
the left represents the total binding energies of different nuclei. The number of nucleons is represented by the x axis and the total binding energy
by the y-axis. Nuclear fusion always produces a nucleus with a larger number of nucleons, so a fusion reaction will only shift the binding energy
toward the positive x-axis. If fusion results in a positive change in binding energy, then there is a significant loss of energy from the nucleus into
the field of the force that keeps it together. An isotope of iron lies at peak of this binding energy curve, so beyond that point fusion will generally
lead to a decrease in the binding energy and general stability of a nucleus. By mass-energy equivalence, a significant loss of energy can result in a
significant loss in mass. Therefore, a fusion reaction yielding an increase in nuclear binding energy also yields a loss of mass. Because mass and energy
must be conserved, this mass defect will lead to a release of energy. This is how the process of nuclear fusion is able to release massive amounts of energy.
The Deuterium-Tritium Cycle: The Most Promising Prospective Fusion Cycle for Reactors
Since any element lighter than Iron can fuse and still release energy, there are many, many more fusion cycles than the basic proton-proton cycle
used in the first section to illustrate conceptual points about fusion energy. For example, given the enormous temperatures, densities, and pressures
accompanying more massive stars, a cycle far more energetic involving fusion reactions on isotopes of Carbon and Nitrogen can replace the proton-proton
as the dominant fusion cycle. With enough time, more average stars can fuse their entire supply of Hydrogen to Helium start a completely different fusion
cycle, called the Triple-Alpha process, ends with the synthesis of Carbon, it's also because of this process that the element most important to the function
of life on Earth, Carbon, is synthesized.
Although there is a wide range of fusion cycles, the one cycle most convenient for use in a reactor on Earth is determinable by the capabilities of
a fusion reactor design to maintain the physical conditions required to ignite the fusion reaction, the relative abundance on Earth of an element to
fuel the cycle, and the actual energy yield of the cycle itself. The most promising candidate to date for a functional Fusion Reactor is a
Deuterium-Tritium cycle ran through a Magnetic Confinement reactor.
Finding deuterium as a fuel source presents no problem as it is found at approximately 1 part in 5000 of Hydrogen in seawater. This adds to
around 10^15 tons of Deuterium present on the planet. Finding Tritium as a fuel source, however, is much more problematic. Tritium is an unstable isotope
of Hydrogen with a half-life of around 10 years so the only way to procure an appreciable amount of Tritium is to breed it from Lithium by neutron
bombardment. The difficulty involved in obtaining Tritium will doubtlessly make it the limiting factor in a fusion reactor's fuel supply.
In order for a future fusion reactor to be self-sustaining, researchers have researched methods of breeding Tritium by capitalizing on loose neutrons
that are released with Helium as part of the Deuterium-Tritium cycle. Since neutrons carry no charge, they will remain unaffected by the a Tokamak
reactor's guiding magnetic fields and are able to escape the plasma. The reactor being constructed at the ITER facility
capitalizes on these loose neutrons by surrounding the main reactor with modules containing Lithium. Neutron bombardment of Lithium has been engineered
as a function of the reactor, so the scarcity of Tritium will present little problems as long as there is a supply of Lithium.

